AI‑driven decision‑making and ever‑tighter release cadences promise faster value delivery. Yet for teams operating under the microscope of regulators, from defence and financial services to healthcare and critical infrastructure, the reality is more complex.
Every change must be validated, every data flow audited and every tool scrutinised for security vulnerabilities. That environment can make even the most straightforward automation initiative feel risky.
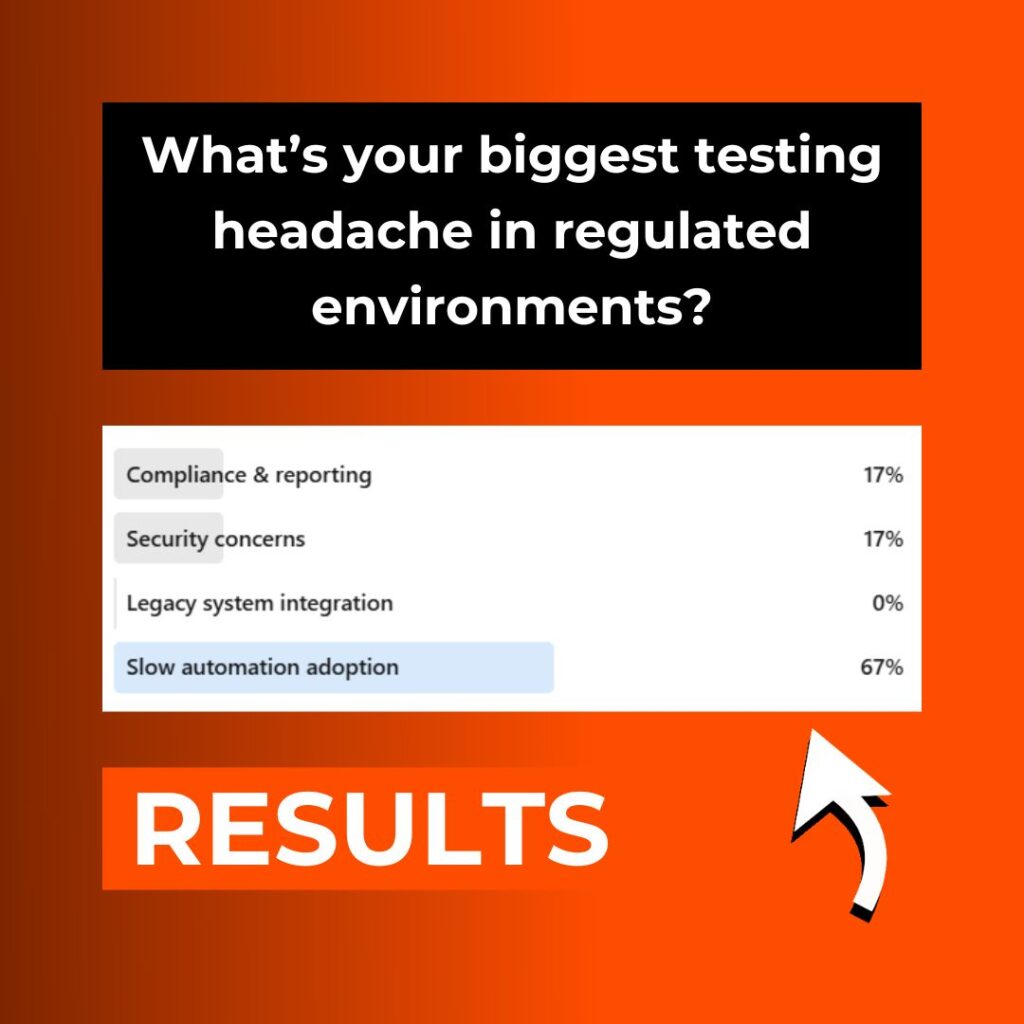
To separate perception from reality we ran a short LinkedIn poll to find out where practitioners are feeling the greatest friction today. Here’s what you told us:
In our recent LinkedIn poll we asked:
“What’s your biggest testing headache in regulated environments?”
The results were striking:
Challenge | Votes |
Slow automation adoption | 67 % |
Compliance & reporting | 17 % |
Security concerns | 17 % |
Legacy system integration | 0 % |
Two‑thirds of respondents told us that, despite all the talk of digital acceleration, getting automation off the ground is still the number‑one blocker. That’s a loud and clear signal that the industry needs practical answers – fast.
Why does automation adoption stall in regulated spaces?
- Regulatory validation overheads
Every script, environment and data source must be auditable. Formal validation packs, change‑control boards and internal QA gates can turn even a small automation pilot into a paperwork marathon. - Risk‑averse culture
Heavily regulated organisations are naturally cautious. A single production incident can have financial, legal and even national‑security repercussions, so teams shy away from change. - Fragmented, legacy tech stacks
Decades‑old mainframes sit alongside modern web and mobile front‑ends. When tools can’t span that mix, progress stalls. - Skills and capacity gaps
Specialist automation engineers are scarce – and the ones you have are buried under day‑to‑day release work. - Uncertain return on investment
Stakeholders want a solid business case before signing off new tooling, yet long validation cycles make early wins hard to prove.
The hidden costs of not automating
- Slower release cycles keep critical updates out of users’ hands.
- Higher operational risk as manual test coverage struggles to keep pace.
- Talent drain when skilled engineers spend nights and weekends repeating the same regression pack.
- Escalating audit overheads as evidence must be captured by hand.
- Put simply, slow adoption is more than an inconvenience – it is a strategic drag on innovation.
What makes the difference?
Requirement | What teams need | How T‑Plan helps |
Cross‑platform reach | Desktop, mobile, web & legacy in one tool | One script runs across Windows, macOS, Linux, iOS & Android |
Fast, validated start‑up | Tool installed, documented and signed off within days | Typical deployment to first test in 15 minutes and repeatable scripts in under four hoursfileciteturn0file0 |
Low‑code empowerment | Business & QA SMEs creating scripts without specialist coding | Intuitive visual interface + Python extension when needed |
Audit‑ready evidence | Tamper‑proof logs, screenshots & reports | Built‑in reporting exports straight to your validation pack |
Award‑winning support | Responsive experts who speak compliance | Dedicated customer success team with 24/5 follow‑the‑sun coverage |
Five practical steps to accelerate adoption today
- Start with a risk‑based pilot – target a high‑value manual regression that always runs late. Demonstrate the time saved and roll the evidence into your business case.
- Automate end‑to‑end, not just the UI – include back‑office or mainframe checks via API, CLI or data‑validation steps so auditors see full traceability.
- Shift validation left – generate your compliance artefacts as the script runs, rather than retro‑fitting them before release.
- Democratise scripting – use low‑code tooling so domain experts can own their tests while engineering focuses on frameworks.
- Measure and broadcast ROI – track cycle‑time reduction, defect‑escape rate and audit hours saved. Share the metrics widely to build momentum.
Ready to move faster?
Slow automation adoption is not an inevitability of working in a regulated industry. With the right partner and the right tooling you can accelerate delivery, reduce risk and keep every auditor satisfied.
If you’d like to see how T‑Plan can have you up and running in minutes – without cutting corners – let’s talk.
Email sales@t‑plan.com or call +44 (0)330 120 0875 to arrange a personalised demonstration.